|
|
Quiz Me! |
Control of Microbial Growth: Physical & Chemical Factors |
|
|
|
Lecture Index | ||
|
|
Course Resources page |
Last revised: Tuesday, February 15, 2000
Reading: Ch. 7 in Prescott et al, Microbiology, 4th Ed.
Note: These notes are provided as a guide to topics the instructor hopes to cover during lecture. Actual coverage will always differ somewhat from what is printed here. These notes are not a substitute for the actual lecture!
Copyright 2000. Thomas M. Terry
1. Overall Effectiveness (from least to most specific)
- Sterilizing Agents-- kill everything (e.g. heat, radiation)
- Disinfectants-- kill most things. Too strong for living tissues (e.g. lysol, NH3)
- Antiseptics-- milder in action. Can be used topically, but not ingested. (e.g. alcohol, iodine)
- Chemotherapeutics-- can be ingested (e.g. penicillin, sulfa drugs)
2. Sterilizing Agents
A. Heat
- Boiling. OK for most food, but not sterilizing. Endospore formers, hepatitis virus can resist.
- Autoclaving. Most common sterilizing procedure. 15 min @ 121 deg. Celsius. Adequate for l liter volumes. Longer times for larger volume.
- Dry Heat. Used for dry products. Typically 170-200 deg. C. overnite.
- Pasteurization. Not a sterilizing treatment, but kills pathogens in milk. 63-67 deg. C. for 30', Now 71 deg. C. for 15 sec.
B. Membrane Filters.
- 0.45 um filters retard bacteria. Good for heat-labile materials. Rapid. But expensive, and filters will clog.
- View examples of membrane filters
C. Chemicals
- Ethylene Oxide = alkylating agent
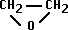
- chemically adds on to proteins, nucleic acids, etc.
- Widely used (in special cabinets) to sterilize heat-sensitive items, e.g. syringes, disposable plastics, rubber items, surgical supplies, etc.
- usually carried out for 1-10 hours at 60 deg. C. Very flammable, must use special precautions. Products retain residues of gases. (e.g. petri dishes have enough to cause mutations in bacteria).
D. Radiation
- UV light. Reacts with DNA, causes DNA damage -- death. Thymine dimers!. But much damage can be repaired, esp. if light available (PHOTOREACTIVATION). NOTE: cannot penetrate glass.
- Ionizing radiation. Gamma rays produce free radicals, destroy all kinds of chemicals. E.g. OH-
3. Disinfectants & Antiseptics (Not mutually exclusive; depends on concentration)
Note: disinfectants are classified into 3 groups:
- heavy metals (Mercury, Silver, Arsenic)- cause protein denaturation
- halogens (Chlorine, Iodine, Hypochlorite)- oxidizing agents. Not usually used as antiseptics, but good for swimming pools, hot tubs, water supplies. Household bleach = 5% soln of hypochlorite- good for all-purpose disinfectant. But don't use with ammonium compounds or acids, can produce explosive gases (nitrogen tricholoride or chlorine gas).
- phenols & cresols- dissolve membranes, denature proteins
- alcohols- denature proteins, dissolve membranes.
- detergents- dissolve membranes
- High level: effective against all life, incl. endospores. E.g. ethylene oxide, 2% glutaraldehyde. May require 10 hours to kill all pop of endospore-forming bacteria
- Intermediate level: defined as tuberculocidal (kill Mycobacterium tuberculosis ), as well as more resistant viruses (hepatitis, rhinovirus). Not effective against endospores.
- Low level: not effective against tuberculosis or endospores, or viruses without membranes. But do kill vegetative bacteria and fungi, used extensively. Economical, not overly toxic to humans. E.g. Lysol, detergents, mercurials.
Take a Self-Quiz on this material
Return to Lecture Index
Return to MCB 229 Course Resources page